Peptide and Antibody Screening
We understand that accurately capturing biomolecular interactions in the lab is challenging. We overcome these challenges by developing a customized protocol for each screening procedure. We use advanced and trusted high-throughput methods including our own novel screening approach with unmatched high sensitivity.

Lead Discovery with Our High-Throughput Screening Methods
Phage display technology is a potent tool in drug discovery, facilitating the development of peptides and monoclonal antibodies that bind with specificity and remarkable affinity to target molecules. This technique involves genetic engineering of bacteriophages, which display foreign peptides or antibodies on viral coat proteins to create a fusion phage. Phage display libraries house an impressive collection of approximately 10^9-10^11 diverse phage clones and serve as an invaluable resource for selecting and identifying polypeptide-based ligands.
Phage Display Affinity Selections
Cell origins carries out both phage display selections and screening of identified peptides and antibodies. In most phage display selection protocols, successive rounds of affinity selections, known as biopanning, are utilized. Here, the library is incubated with the target antigen and bound phages are collected by elution. Selected peptides or antibodies are identified and sorted by next-generation sequencing (NGS) and bioinformatic analysis, respectively. At Cell Origins, we use multi-tier phage display selection techniques to identify peptides and monoclonal antibodies with optimal binding kinetics and pharmacokinetics, ensuring that lead compounds maintain both high binding affinity and specificity, while minimizing off-target effects. Learn more about our phage display biopanning strategies and how we can optimize your affinity selections.
Peptide and Antibody Screening
Following phage display affinity selections, the peptide and antibody hits are subjected to screening to identify the best leads. The screening of selected peptides and antibodies typically involves biomolecular interaction analysis. Such interactions are analyzed by measuring the physical contact between the biomolecules utilizing various methods that include, but are not limited to:
- Peptide- and antibody-arrays
- Enzyme-linked immunosorbent assay (ELISA)
- AlphaLISA
- Surface plasmon resonance (SPR)
- Dynamic light scattering (DLS).
Biomolecular interactions between proteins, nucleic acids, carbohydrates, and lipids play important roles in most biological processes. Our scientists know how difficult these interactions can be to recapture in the laboratory, which unfortunately causes many screening procedures to fail and to misidentify or lose lead peptide and antibody molecules. At Cell Origins, we therefore develop a
customized protocol for each screening procedure to ensure that you will identify the most optimal lead molecules. Oftentimes, our screening methods utilize not only purified target antigen, but also incorporate cell and tissue analyses to provide the most accurate results.
Increased Assay Sensitivity with Phage Display Screening
Whereas phage display affinity selections (biopanning) are typically completed in a time-efficient manner, screening of the selected peptides and antibodies using biomolecular interaction analysis often becomes a bottleneck. This is caused by time-consuming and expensive synthesis, production, and purification steps of the peptides and antibodies. Additionally, soluble peptides and antibodies exhibit limited stability under storage, which can complicate the screening procedures and add to the cost of the experiment.
Ampli Φ
Traditional methods of biomolecular analysis, like ELISA, have been widely used but are often inadequate in achieving the required level of assay sensitivity. This limitation hinders accurate and precise analysis of biomolecules due to measurements with low signal-to-background ratios. ELISA's sensitivity shortcomings can result in a compromised ability to detect low concentrations of analytes, leading to potential false negatives or missed detections. Additionally, the limited dynamic range of many assays can restrict the ability to accurately quantify biomolecular interactions at both high and low concentrations, limiting the scope of analysis. As a result, it is imperative to choose a method of analysis with improved assay sensitivity, wider dynamic range, and greater precision for biomolecular analysis.
At Cell Origins, we understand that not only should peptide and antibody screening procedures be carried out with the most accuracy and precision, but also in the most time-efficient and cost-effective manner. Therefore, we are proud to offer our own novel approach to high-throughput screening of phage-displayed peptides and antibodies called Ampli Φ (patent pending). Our method guarantees rapid production and purification of phage particles followed by biomolecular interaction analysis with greatly enhanced signal amplification compared to conventional biomolecular interaction analyses methods such as ELISA (Figure 1).
In the field of drug discovery, the ability to accurately measure and characterize biomolecular interactions is of utmost importance, as it allows researchers to identify potential therapeutic targets, validate drug candidates, and gain a deeper understanding of the underlying mechanisms. Our novel Ampli Φ technique offers enhanced accuracy and precision by amplifying the measured signal and can be used over a wide range of concentrations. Compared to traditional techniques such as ELISA, the Ampli Φ method boasts a remarkable signal amplification of 40-100 times, providing a significant enhancement that guarantees more reliable results (Figure 2). This level of sensitivity and specificity is crucial in the drug discovery process of peptides and antibodies.
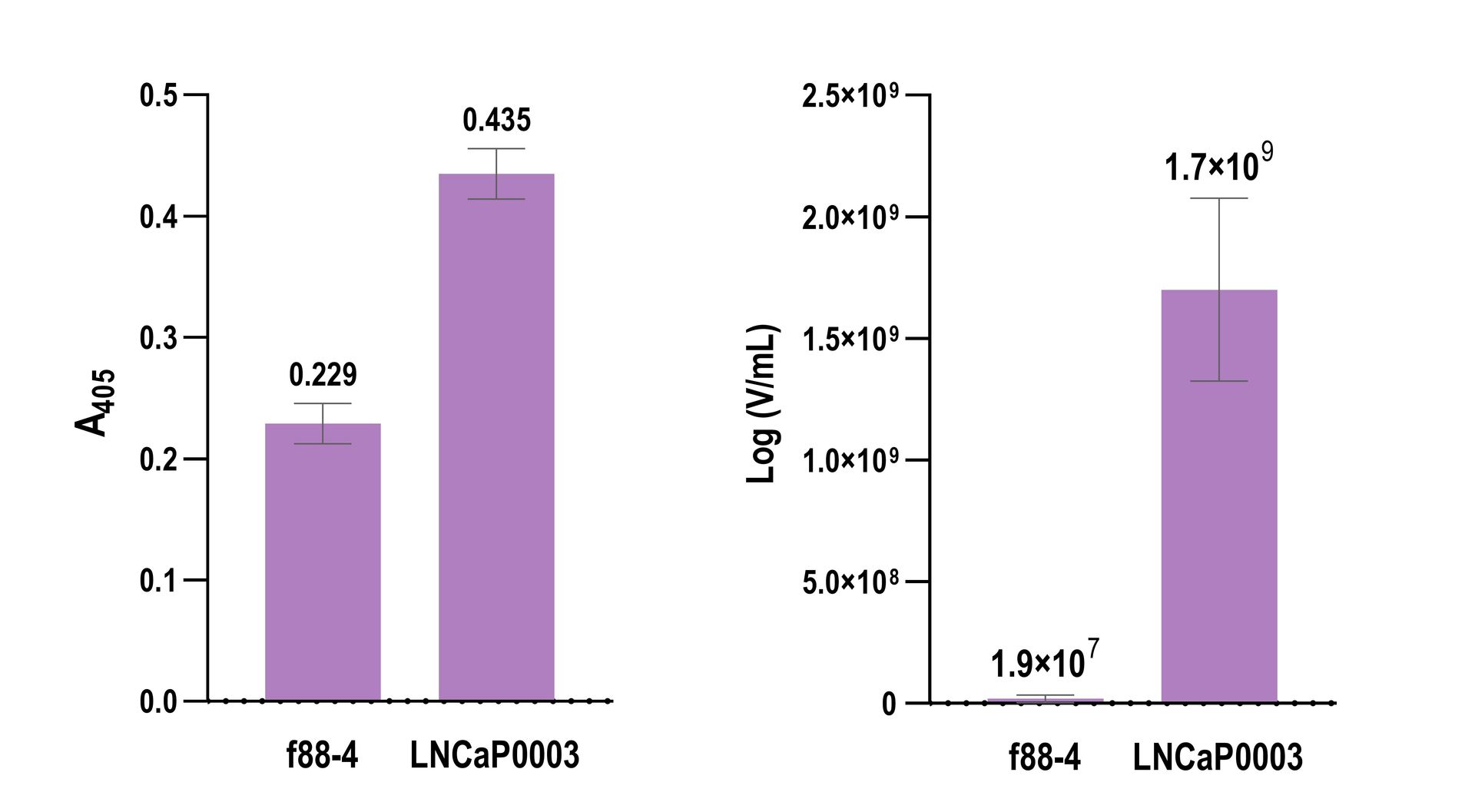
Figure 1. Peptide- or antibody-displaying phage may be used in biomolecular interaction studies instead of soluble peptides or antibodies. However, traditional methods of biomolecular analysis such as ELISA typically fail to provide ample sensitivity, which results in low signal-to-background measurements. Instead, signal amplification may be achieved via Cell Origin's Ampli Φ method that greatly enhances the signal-to-background ratio. (Left) Biotinylated f88-4 phage (control without a displayed peptide) and LNCaP0003 phage known to bind the prostate cancer cell line LNCaP were incubated with the cells. The binding of each phage clone was probed by horseradish peroxidase (HRP)-conjugated streptavidin and measured spectrophotometrically at 405 nm after the addition of a HRP substrate. (Right) Phage clones, f88-4 and LNCaP0003 were incubated with LNCaP cells under identical conditions to the described ELISA experiment. Bound phages were eluted using HCl and quantified using qPCR.
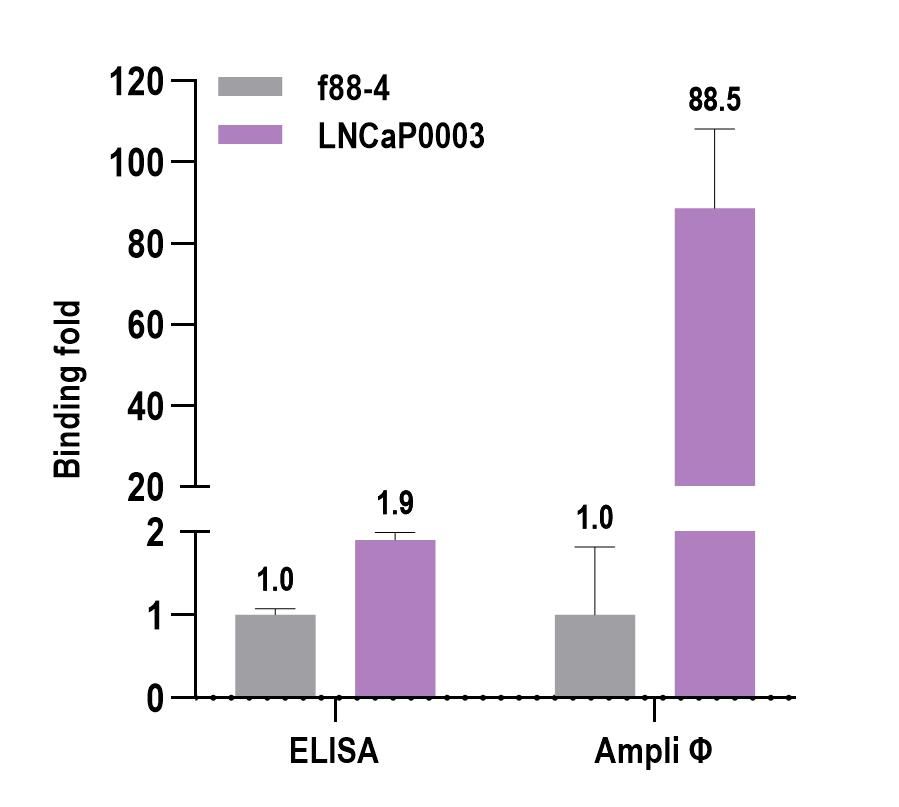
Figure 2. Traditional biomolecular analysis methods like ELISA often lack the required assay sensitivity, leading to low signal-to-background ratios. This limitation hampers the accurate detection of analytes, potentially resulting in false negatives. Here, both ELISA and our Ampli Φ method were used to measure the binding of phage clones f88-4 (control without a displayed peptide) and LNCaP0003 that is known to bind the LNCaP cell line. As expected, both methods were able to differentiate the binding of f88-4 and LNCaP0003 to the cell line. However, the signal-to-background of the ELISA was merely 1.9, whereas the Ampli Φ method enhanced this ratio approximately 40 times to 88.5.
Our Expertise in Peptide and Antibody Screening
Our experienced scientists are acclaimed experts in phage display technology and screening of soluble peptides and antibodies, as well as peptide- and antibody-displaying phage particles. In fact, the scientists at Cell Origins have published numerous articles in the field and developed novel methods of biomolecular interaction analyses, of which some are patent pending. We consistently push the boundaries to achieve unmatched sensitivity of our customized screening assays so you can quickly identify lead molecules while keeping costs down.
We are dedicated to supporting you throughout your entire journey of peptide or antibody discovery. By understanding your unique experimental challenges and obstacles, we offer in-depth research analysis and a tailored project strategy. The screening procedures take place in our cutting-edge laboratories, and we consistently provide you with comprehensive reports that empower you to make informed decisions in transforming your hits into leads.
Contact Us to Learn More
Cell Origins is at the forefront of utilizing advanced peptide and antibody screening methods. Our groundbreaking work includes the development of novel screening methods with unmatched sensitivity. We invite you to reach out to discover how our innovative screening platforms can accelerate your drug discovery journey.

