Customized Phage Display Libraries
Our custom libraries are made with built-in options for post-selection analysis such as biotinylation, expression of soluble ligands, affinity isolation, and more. This allows you to get the most out of your peptide or antibody phage display library and get the best results. Our experts are here to help you every step of the way, ensuring that you get the most optimal phage display library for your needs.

Leading Experts in Phage Display
At Cell Origins, we specialize in creating custom peptide and antibody phage display libraries to maximize the potential of your selections. Our dedicated team collaborates with you to design and construct a customized library tailored to your unique requirements.
By incorporating selection and tagging strategies directly into the phage display libraries, we facilitate the identification of peptides and monoclonal antibodies with superior affinity, specificity, as well as other essential properties for both
in vitro and
in vivo applications. Moreover, these integrated features enable efficient post-selection analysis, including high-throughput tagging and screening, ensuring rapid and efficient results.
Endless Possibilities with Customized Libraries
Phage display can be used for a variety of applications at different stages in drug development including peptide and antibody discovery, drug candidate screening, and lead optimization. Phage display technology is versatile because almost any type of peptide or antibody library can be screened against any type of target. Additionally, the phage display platform is highly efficient and can be used to screen billions of different peptides or antibodies in a single day. This high throughput capacity makes phage display an attractive technology in drug discovery. However, some important considerations need to be made before choosing which type of library to use for your phage display selections.
Choosing the Right Phage Display Format
The structure of the filamentous phage offers many opportunities for the display of polypeptide-based ligands such as peptides, antibodies, antibody fragments, vhh/nanobody, and more. While the expression of foreign ligands on coat proteins pIII and pVIII is the most common, the utilization of each coat protein presents its own advantages.
pIII
Coat protein III (pIII) is located at one end of the phage virion and acts as the f-pili-binding protein during infection of E. coli. This process is crucial for the successful infection of E. coli and ultimately, for their propagation. Despite its key involvement in bacterial infection, pIII can successfully be used for display without significantly affecting the phage lifecycle. This property ensures the propagation of each clone in the phage display library, maintaining the sequence diversity of the library. For this reason, pIII is the most commonly used display format in phage display technology.
pVIII
Coat protein VIII (pVIII), also known as the major coat protein, is expressed in over 2000 copies, providing ample opportunities to display multiple copies of a peptide. However, not all 2000 copies are typically used for phage display. Rather, only ~10% of the pVIII copies are used for expression. The display of large proteins such as full-length monoclonal antibodies and antibody fragments is challenged due to the impairment of virion release from E. coli during the phage lifecycle. Nevertheless, certain types of peptide selection strategies, such as those that require the binding of polyvalent antigens.
pVI and pVII
Coat protein VI (pVI) is adjacent to pIII. While comparatively less common, pVI display formats have proven useful for expressing larger proteins at both the N- and C-termini. Notably, this type of display has been used to create cDNA libraries and investigate protein-molecule interactions. Coat protein VII (pVII) is located at the opposite end of the virion. Display using pVII has been gaining popularity lately, particularly for expressing antibodies and antibody fragments. Moreover, using pVII display in conjunction with other display techniques can streamline tagging and post-screening analysis.
pIX
Recent advancements have significantly enhanced the display on coat protein IX (pIX) through the utilization of diverse phagemid systems and signal sequences. However, when compared to other systems, particularly those employing pIII, the expression levels of foreign polypeptides on pIX tend to be lower. Nevertheless, this lower expression level increases the likelihood of monovalent display, thereby facilitating the selection of high-affinity ligands. This progress underscores the importance of optimizing the display system for continuously improved results.
Choosing an Optimal Expression System
Dr. George P. Smith pioneered the development of phage display technology in 1985 at the University of Missouri. His groundbreaking work involved genetically modifying the filamentous phage ssDNA genome to express foreign peptide sequences on coat protein III (pIII). Expanding on this innovation, large random phage libraries were created, leading to the development of biopanning for the selective identification of highly specific peptides and antibodies. Today, two distinct types of vectors are commonly utilized for the display of foreign polypeptide-based ligands.
Bacteriophage Vectors
Filamentous bacteriophage vectors (Fd and M13) encode a modified version of the complete phage genome. Alongside the wild-type genes, most bacteriophage vectors have an antibiotic selection gene. Additionally, they carry a recombinant hybrid gene that encodes the foreign peptide or antibody fused to the coat protein utilized for display. Expression of both the wild type and hybrid genes facilitates a lower level of foreign polypeptide display, which bears the advantage of enabling the selection of high-affinity polypeptide. However, bacteriophage vectors are not be ideal for displaying antibodies or antibody fragments due to their large size, which impedes E. coli infection and complicates transformation. Nevertheless, bacteriophage vectors have proven successful in peptide phage display. A notable advantage is that these vectors do not necessitate the use of helper phages for phage particle propagation, as their genome encompasses all the required replication genes.
Phagemids
Phagemids are vectors based on plasmids that contain phage and bacterial origins of replication, along with an antibiotic selection gene. Moreover, a phagemid vector typically encodes a coat protein fused to a foreign DNA sequence. This fusion is used for the display of monoclonal full-length antibodies, antibody fragments, nanobodies, or peptides. Phagemid vectors do not rely on helper phage proteins to replicate in
E. coli. However, helper phages are indispensable for producing phage clones, as they carry essential genes required for the assembly and release of the phage particles. The small size of phagemids facilitates hassle-free transformation in E. coli. The efficiency of
E. coli transformation directly impacts the diversity of the phage display library, making it a crucial consideration when selecting a suitable vector. Additionally, the small size of phagemid vectors allows for easy manipulation using recombinant DNA technology.
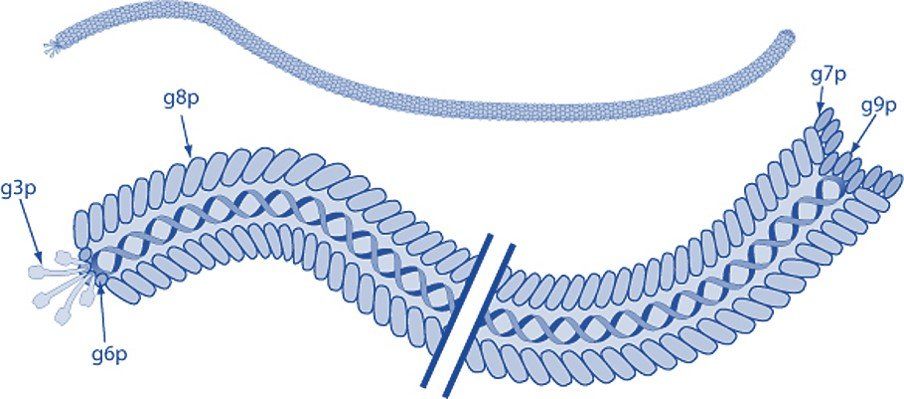
Filamentous bacteriophage express five coat proteins that can each be used to display foreign polypeptides and employed in phage display technology. Coat protein VIII (pVIII) is the major coat protein and is expressed in >2000 copies along the virion. Coat proteins III and VI are expressed at one tip of the bacteriophage, while pVII, and p IX are located at the opposite end.
Modified from ViralZone, SIB Swiss Institute of Bioinformatics. Permission to use ViralZone graphics in Wikimedia Commons kindly granted by SIB (legal(at)sib.swiss), 2021-02-01. -
https://viralzone.expasy.org/resources/Inoviridae%5Fvirion.jpg on https://viralzone.expasy.org/113, CC BY-SA 4.0,
https://commons.wikimedia.org/w/index.php?curid=99827306
Phage Display Peptide & Antibody Libraries
Peptide phage display technology is an efficient technology with a broad range of applications in research and drug discovery. Peptides can serve as drugs to induce therapeutic effects or transport cargo to target disease biomarkers. The compact nature of peptides presents numerous benefits, such as their ability to penetrate tissues and be easily eliminated from the body. These advantages are particularly crucial in cancer radiotherapy, where precise delivery to target cells and swift clearance are vital to prevent damage to non-targeted tissues. Antibody phage display libraries are invaluable tools in the field of drug discovery and scientific research. These versatile libraries facilitate the screening of antibodies and peptides, identifying those with exceptional binding affinities and specificities. With the ability to target a diverse array of antigens, including proteins, peptides, small molecules, and even whole cells and tissues, these libraries offer an enormous potential for advancing biomedical knowledge.
Let's Get Started!
At Cell Origins, we closely collaborate with you to create a personalized peptide or antibody phage display library tailored to your unique objectives. Our dedicated team of experts incorporates state-of-the-art selection and tagging strategies into the library to enable the identification of ligands with exceptional binding kinetics and pharmacokinetics. This streamlines the process of screening, ensuring accuracy and reliability of your phage display selections. Throughout the development of your customized phage display library, our team provides comprehensive support and protocols. Whether you prefer to schedule a meeting or reach out to us directly, we are eager to learn more about your research needs. Trust us to develop a custom phage display library that meets your goals.